top of page
%2C_Dhanbad_Logo.png)
NRL Research Group
Current research focus
ONCOLOGY DRUG DISCOVERY
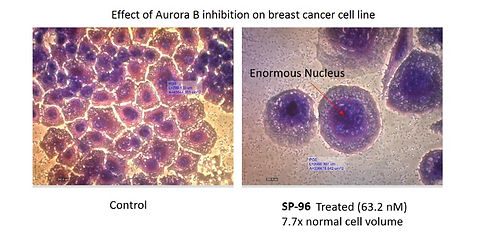
The ongoing research in our lab aims to identify novel small molecule inhibitors as targeted therapies. We target key oncogenic kinases including FLT3, RET, Mps1, Aurora B, and TNIK, which are implicated in aggressive cancers such as triple-negative breast cancer (TNBC), non-small cell lung cancer (NSCLC), and thyroid carcinomas.
Our approach integrates structure-based drug design using computational tools such as molecular dynamics simulations and free energy perturbation studies, followed by the synthesis of the designed compound libraries using heterocyclic chemistry, and biological evaluation using various enzymatic and cellular assays. Emphasis is placed on structure-activity relationship driven lead refinement.
In addition to monotherapy development, we investigate rational drug combinations to identify synergistic interactions and overcome resistance mechanisms. Expanding beyond kinase inhibition, we are also exploring ferroptosis induction via GPX4 inhibition as an emerging therapeutic strategy. Further, we employ bioinformatic tools for target validation by studying the expression of various cancer targets in specific cell lines.
Selected Publications:
-
Lakkaniga, N. R., Zhang, L., Belachew, B.A., Naresh, G., Frett, B., Li, H.Y. Discovery of SP-96, the first non-ATP-competitive Aurora Kinase B inhibitor, for a reduced myelosuppression profile. European Journal of Medicinal Chemistry 2020, 203, 112589. https://doi.org/10.1016/j.ejmech.2020.112589
-
Zhang, L., Moccia, M., Briggs, D.C., Bharate, J.B., Lakkaniga, N.R., Knowles, P., Yan, W., Tran, P., Kharbanda, A., Wang, X. and Leung, Y.K., 2022. Discovery of N-Trisubstituted Pyrimidine Derivatives as Type I RET and RET Gatekeeper Mutant Inhibitors with a Novel Kinase Binding Pose. Journal of Medicinal Chemistry, 65(2), pp.1536-1551.
-
Lakkaniga, N. R., Gunaganti, N., Zhang, L., Frett, B., & Li, H. Pyrrolo[2,3-d]pyrimidine derivatives as inhibitors of RET: Design, synthesis and biological evaluation. European Journal of Medicinal Chemistry 2020, 206, 112691. https://doi.org/10.1016/j.ejmech.2020.112691
ANTI-VIRAL DRUG DISCOVERY
In collaboration with Scripps Research, our lab focuses on identifying novel small molecule group-2 hemagglutinin inhibitors as potential antiviral agents. Hemagglutinin (HA)—a key surface glycoprotein of the influenza virus responsible for viral attachment and entry into host cells. By interfering with HA-mediated membrane fusion, we aim to block the initial step of viral infection, providing a promising strategy to combat both seasonal and pandemic influenza strains.
We employ an interdisciplinary approach combining computational modeling, biochemical assays, X-ray crystallography, and structure-based drug design to identify and optimize small-molecule inhibitors of HA. Our work contributes to the global effort to develop broad-spectrum antivirals with the potential to overcome resistance associated with current therapies.
COMPUTATIONAL STRUCTURAL BIOLOGY
Our lab also investigates protein structure and function through computational structural biology. We employ enhanced sampling molecular dynamics (MD) simulations to explore the conformational landscapes of proteins of our interest, enabling the identification of unresolved druggable conformations and allosteric binding pockets critical for drug design. These simulations offer mechanistic insights into dynamic biological and biochemical processes, including protein activation, ligand binding, and allosteric regulation.
Selected Publications:
-
Singh, A. and Lakkaniga, N.R. (2025). Exploring the Conformational Space of MPS1 Kinase Using Metadynamics. Proteins: Structure, Function, and Bioinformatics, 93: 1118-1127. https://doi.org/10.1002/prot.26796
-
Lakkaniga, N. R.; Balasubramaniam, M.; Zhang, S.; Frett, B.; Li, H.-Y. Structural Characterization of the Aurora Kinase B “DFG-Flip” Using Metadynamics. The AAPS Journal 2020, 22 (14). https://doi.org/10.1208/s12248-019-0399-6
EVALUATION AND FORMULATION OF PHYTOCONSTITUENTS FOR THERAPEUTIC APPLICATIONS
In collaboration with Emami Ltd., we explore the therapeutic potential of various phytoconstituents through comprehensive pharmacological evaluation and advanced formulation strategies. Selected natural compounds are screened for anti-inflammatory, antioxidant, antimicrobial, and anticancer activities using in silico models.
Selected Publications:
-
Chatterjee, A., Jayaprakasan, M., Chakrabarty, A. K., Lakkaniga, N. R., Bhatt, B. N., Banerjee, D., ... & Dubey, S. K. (2024). Comprehensive insights into rheumatoid arthritis: Pathophysiology, current therapies and herbal alternatives for effective disease management. Phytotherapy Research.

bottom of page